BOMBA “Nanoformas tóxicas dentro de la vacuna Pfizer-Biontech Covid”.
La investigación fue publicada por US Journal of Virology y Disinfection Italian medical review. El conocimiento de la peligrosa toxicidad de estas nanoformas, de hecho, podría configurar el “fraude"
¡Exclusivo! “Nanoformas tóxicas dentro de la vacuna Pfizer-Biontech Covid”.
La investigación fue publicada por US Journal of Virology y Disinfection Italian medical review
25 de marzo de 2023
por Fabio Giuseppe Carlo Carisio
«Igualmente no conforme, y en conflicto con la práctica regulatoria-toxicológica ahora consolidada relacionada con las nanoformas, la Sección 11 (Información toxicológica) de la Hoja de datos de seguridad de Pfizer-BioNTech, con referencia al producto Comirnaty dice: “Las propiedades toxicológicas no han sido investigado a fondo (Figura 10)”».
¡Lo que fue informado exclusivamente por una investigación de Gospa News en febrero de 2022 ahora se eleva a la dignidad de una publicación científica internacional e incluso termina en la denuncia de un sindicato de la Policía Estatal!
Sin conocerse en Italia, el periodista ( que ahora escribe) y el bioquímico Gabriele Segalla llegaron al mismo sorprendente descubrimiento en tiempos casi idénticos al analizar la documentación confidencial divulgada por la farmacéutica de Nueva York para obtener la autorización para el uso de emergencia de Sueros de genes de ARNm para Covid por la Administración de Alimentos y Medicamentos (FDA), el regulador de medicamentos estadounidense.
Para Gospa News es la mejor prueba de la solidez de nuestras investigaciones científicas que se echan de cara a los Fact-Checkers pagados por la Unión Europea , donde se entrelazan los intereses del Grupo Bilderberg con los de la universidad de la que es titular el actual Ministro. de Salute Orazio Schillaci, por lo que hemos sido baneados u oscurecidos en varias Redes Sociales (Facebook, LinkedIn, YouTube).
Para el investigador lombardo Segalla, director ejecutivo y científico jefe de Multichem R&D Italia (Rozzano, Milán), ¡ es un récord mundial científico en el campo de las vacunas!
Su investigación, “realizada por razones científicas y éticas” por un académico independiente sin financiación alguna, se publicó en Quaderno el 2 de octubre de 2022 de la revista científica italiana Disinfection y posteriormente tuvo un gran eco con la publicación revisada en International Journal of Vaccine en USA (con sede en Dallas, Texas) el 26 de enero de 2023. El autor es Doctor en Química Pura (Química Orgánica-Biológica), especialista en Química de microemulsiones y sistemas coloidales.
Hasta ahora, de hecho, ningún científico había descubierto en un único estudio publicado hasta 4 causas diferentes y desconocidas de la criticidad toxicológica de las nanopartículas lipídicas utilizadas como vectores del antígeno mRNA de la proteína Spike PS2 (en sí misma altamente tóxica como se ha establecido ) . por otras investigaciones previas).
Los médicos del Hospital Universitario Thomas Jefferson de Filadelfia habían constatado, con un estudio empírico en ratones, el peligro altamente inflamatorio de estos nanomateriales por su riesgo de “inhibir” o “alterar” las respuestas inmunitarias de forma “duradera” con la riesgo de daño hereditario. Los investigadores obviamente se advirtieron a sí mismos al decir que las reacciones adversas en animales de laboratorio más similares a los humanos en su composición genética no necesariamente habrían ocurrido también en las personas.
Pero bastó para lanzar una alarma inquietante que hoy el estudio del bioquímico italiano no solo afianza sino que detalla la probable patogenia (el origen fisiológico) de estos efectos indeseables.
DENUNCIA DEL SINDICATO POLICIAL SOBRE LA “DROGA IMPERFECTA”
No solo. Gabriele Segalla señaló, entre los sensacionalistas y repetidos incumplimientos de las normas farmacológicas sobre el análisis de la genotoxicidad, cometidos con total indiferencia del regulador de medicamentos de la UE, la Agencia Europea de Medicamentos (EMA), también probables violaciones de relevancia penal por miembros del suero del gen del ARNm de Comirnaty que lo hacen “inestable, ineficaz e inseguro”, configurando así la definición de “droga imperfecta” cuya comercialización y administración está prohibida en Italia por el artículo 443 del Código Penal.
¡Pero eso no es todo! Al cruzar la investigación bioquímica de Comirnaty con las de otra patente, Biontech descubrió que esta compañía farmacéutica estaba al tanto de “la “ toxicidad elevada” atribuida a los “liposomas y lipoplejos con carga positiva” se refiere a “formulaciones de ARN encapsuladas en nanopartículas de lípidos catiónicos”. —de la misma categoría que las utilizadas en Comirnaty— y denominadas, en este contexto, “lipoplexes”».
Por ello, el explosivo estudio fue adquirido como Asesoría Técnica del Partido en un memorando complementario en las denuncias interpuestas por la abogada Antonietta Veneziano en el Ministerio Público de Catanzaro a nombre de Antonio Porto, como Secretario General Provincial Caserta de la Organización Libertad y Unión de Seguridad (LES) de la Policía del Estado, así como el Secretario General Regional de Campania, y por el Excmo. Bianca Laura Granato, ex Senadora de la República Italiana.
El conocimiento de la peligrosa toxicidad de estas nanoformas, de hecho, podría configurar el “fraude potencial” que, de comprobarse, podría otorgar mayor gravedad a los casos de lesiones graves o letales relacionadas con reacciones adversas a este suero del gen del ARNm.
Entre ellos, cabe mencionar sobre todo aquellos con consecuencias cancerígenas que ya han sido tratados en una sentencia de la jueza Susanna Zanda del Tribunal Civil de Florencia enviada a la Fiscalía de Roma para investigaciones sobre posibles implicaciones penales.
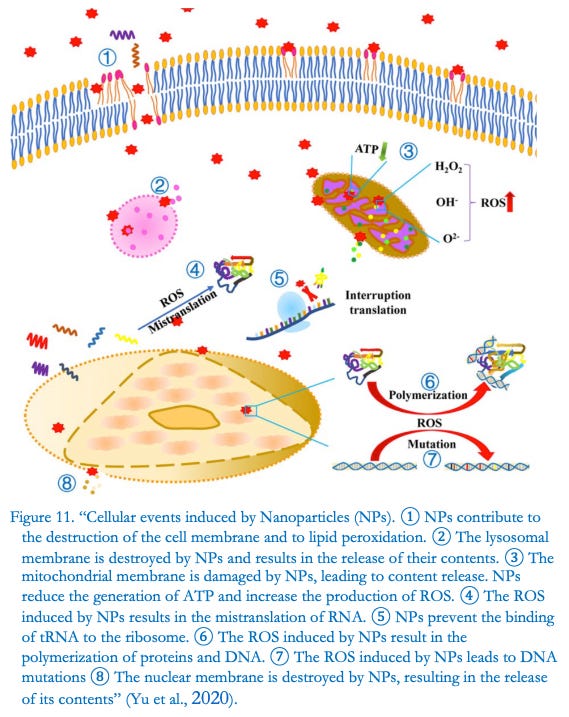
EL FENÓMENO ROS PROVOCA DISFUNCIONES EN LOS ÓRGANOS HUMANOS
En resumen, hemos resumido lo que hace que la investigación realizada en los laboratorios de I+D de Multichem en Rozzano sea única, excepcional y disruptiva. Ahora veamos en detalle la “Criticidad químico-física y el potencial toxicológico de los nanomateriales lipídicos contenidos en una vacuna de ARNm COVID-19” citando el título exacto del estudio.
La investigación es de tal complejidad y abundancia de detalle científico que nos obliga a realizar un análisis basado esencialmente en el resumen y en las conclusiones. Profundizando sólo en algunos aspectos de la misma.
«El preparado medicinal denominado Comirnaty de Pfizer-BioNTech es una dispersión acuosa de nanomateriales lipídicos, destinado a constituir, tras su descongelación y dilución, el producto acabado para inyección intramuscular. En el presente estudio, examinamos algunas criticidades físico-químicas evidentes del preparado, en cuanto a la inestabilidad manifiesta de su composición cualitativa-cuantitativa, así como su consecuente potencial toxicológico, en este caso relacionado con la posible formación de ROS (especies reactivas de oxígeno). ), tras la inoculación intramuscular, en diferentes sitios biológicos, como, potencialmente, riñones, hígado, corazón, cerebro, etc., provocando disfunciones y alteraciones de los mismos»
Así escribe la Dra. Segalla en el resumen, recordando la investigación de la Universidad de Lund (Malmoe, Suecia) que había realizado «el primer estudio in vitro sobre el efecto de la vacuna de ARNm COVID-19 BNT162b2 en la línea celular de hígado humano». » presentando «evidencia de entrada rápida de BNT162b2 en las células y posterior transcripción inversa intracelular de ARNm de BNT162b2 en ADN«.
Las ROS son partículas que contienen oxígeno, entre las que destacan el peróxido de hidrógeno (H2O2), el radical anión superóxido (O2-) y los radicales hidroxilo (•OH). Se producen predominantemente en orgánulos celulares como el retículo endoplásmico (RE), los peroxisomas y, en particular, en las mitocondrias.
El fenómeno ROS descubierto por el bioquímico italiano podría estar en el origen mismo de graves consecuencias.
De hecho, remarca sobre el estudio italiano: « Los estudios de carcinogenicidad, genotoxicidad y mutagenicidad del preparado no se han realizado con el consentimiento del organismo certificador , aunque ahora ha sido confirmado por numerosos estudios científicos que Reactive Oxygen Species (ROS), generada por nanopartículas, puede tener un alto potencial cancerígeno, genotóxico y mutagénico».
Mientras que: «Además, Yu et al. señalan que la extrema penetración y movilidad de las nanopartículas dentro del cuerpo explican su fácil entrada a la circulación sistémica y acumulación en los órganos».
Even a recent search of scientific literature published by a group of researchers at the Superior Institute of Health in Rome had to admit that «biodistribution studies, as in ref. [103], on liposome microparticles (LNP) showed that the material does not stop at the inoculation site» as it should have done.
This was also attributed by a genomics expert and an American molecular biologist to the very high concentration of plasmids (DNA molecules) of the toxic Spike protein in the gene serums which, spreading in various parts of the human body, are able to reproduce for months .
THE MYSTERIOUS AND DANGEROUS “ALC” EXCIPIENTS
«Of particular concern is the presence in the formulation of the two functional excipients, ALC-0315 and ALC-0159, neverused before in a medicinal product, nor registered in the European Pharmacopoeia, nor in the European C&L inventory. The current Safety Data Sheets of the manufacturer are omissive and non-compliant, especially with regard to the provisions of current European regulation on the registration, evaluation, authorization and restriction of nanomaterials».
On this topic, Segalla’s research highlights a series of numerous pharmaceutical “critical issues and drawbacks” of the “Comirnaty COVID-19 mRNA BNT162b2vaccine, in its original version and composition, called PBS/Sucrose”.
The two functional excipients responsible for the formation of lipid nanoparticles, ALC-0315 and ALC-0159, are not registered in any Pharmacopoeia, nor are they among the substances examined and classified in accordance with Regulation (EC) No1272/2008on classification, labelling,and packaging of substances and mixtures in Europe(CLP).
These excipients also do not appear in the inventory of substances registered in accordance with Regulation (EC) No1907/2006concerning the Registration, Evaluation, Authorisation,and Restriction of Chemicals in Europe(REACH). Therefore, their toxicological profile is not known in thefirst place.
Precisely these two components had been the subject of a sensational complaint in the United States of America.
Lieutenant Colonel Theresa Long, brigade surgeon for the 1st Aviation Brigade Ft. Rucker, Alabama, with experience also in the military bacteriological center of Fort Detrick, to oppose the dangerous compulsory vaccination ordered by the Department of Defense also for pilots, he made an affidavit, a sworn expert report, according to the American legislation that protects “whistleblowers” when they report crimes within the public administration.
In it she highlighted multiple criticalities of Pfizer-Biontech and Moderna’s mRNA gene serums. Among these also the dangerousness of the two excipients.
«My assessment is that ALC 0315 is a known toxin with little study, specifically it is still lacking toxicity, carcinogenic, and teratogenic studies and is specifically restricted to “research only” and effectively has no prior use history, with the SDS designation of (GHS02), listed as H315 and H319, in other words, hazardous if inhaled, ingested or in contact with skin and a health hazard with the designation (P313). A review of the SDS outlines that it is not for human or veterinary use» Dr. Long wrote in her affidavit.
The opinion of the lieutenant colonel of the US Air Force, however, remained embedded in a military report that assumed media visibility but not scientific dignity.
This is why Dr. Segalla deserves great credit for having signaled this toxicological risk to the entire medical community through an official published study.
THE SERIOUS FAILURE OF PFIZER AND EMA ON NANOFORMS
But it’s not over. In the research of the Italian biochemist, a series of serious failures committed by the Big Pharma producers of the mRNA gene serum and ignored by the regulatory bodies are listed.
Not all the chemical-physical analysis procedures and toxicological testsrequiredfor nanoforms of these substanceshave been carried out, contrary to Regulation (EU) 2018/1881amending Regulation (EC) No 1907/2006 of the European Parliamentand of the Council concerning the Registration, Evaluation, Authorisation,and Restriction of Chemicals (REACH),to include the nanoforms of substances.
The Safety Data Sheets of the Comirnaty preparation do not report information on the characteristics of the nanoforms present in the composition of the preparation itself, contrary to the provisions of the aforementionedRegulation (EU) 2018/1881and Regulation (EU) 2020/878.
The actual values of the Polydispersityindex and the Zeta potential of the nanoparticles present in the preparation are unknown. This leads to absolute uncertaintyin the determination of the chemical-physical stability of nanoparticles and their aggregates, with consequent unpredictability inherent both to the potential efficacy of the vaccine and to the degree of penetrability and mobility of its nanoparticles within the human body, as well as their possible entry into the systemic circulation and accumulationin organs such as kidneys, liver, heart, brain, lungs
«The analysis of the characteristics of nanoparticles (size, total surface area, state of aggregation or agglomeration, polydispersityindex, surface charge, etc.), as already described above and as expressly reiterated inthe aforementioned regulations, isessential in order to determine their possible cytotoxic, genotoxic, mutagenic and carcinogenic potential» explains Segalla in the text of his study.
Despite this, EMA, in its report dated 19 February 2021,regarding the assessment ofthe Comirnaty vaccine, writes:
No genotoxicity nor carcinogenicity studies have been provided. The components of the vaccine formulation are lipids and RNA that are not expected to have genotoxic potential.(EMA/707383, 2021, p. 55)As per guidance, no genotoxicity nor carcinogenicity studies were performed. The components of the vaccine (lipids and mRNA) are not expected to have genotoxic potential. This is acceptable to the CHMP.5[my emphasis](EMA/707383, 2021, p. 56).
Committee for Medicinal Products for Human Use (CHMP) is the European Medicines Agency responsible for preparing the agency’s opinions on all matters relating to medicinal products for human use. And he considers the mere “prediction” that the components of the gene serum are not genotoxic to be “acceptable” without evidence.
This appears to contradict the sentence published in the incipit of the article expunged from the Pfizer-BioNTech Safety Data Sheet, in reference to the Comirnaty product, which explicitly states: “Toxicological properties have not been thoroughly investigated (Figure 10)”.
This should not, unfortunately, be too surprising given that for many years the European Commission has been funding massive experimentation in the biomedical field of nanoparticles of various kinds, lipid and synthetic, even with the use of the very dangerous and infamous graphene oxide (detected by some Spanish and Italian research in Pfizer gene serum or in vaccinated people), and therefore his laxity in avoiding demanding genotoxicity tests which, like other studies, could have highlighted carcinogenic risks is understandable.
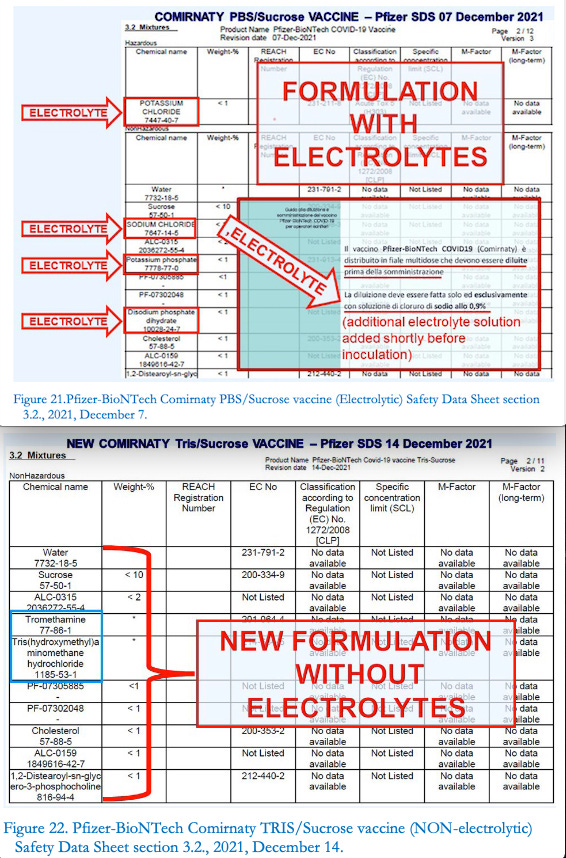
THE ELECTROLYTES DISAPPEARED FROM THE NEW COMIRNATY
«The presence of electrolytes in the preparation and the subsequent dilution phase after thawing and before inoculation raise well-founded concerns about the precarious stability of the resulting suspension and the polydispersityindex of the nanomaterials contained in it, factors that can be hypothesized as the root causes of numerous post-vaccination adverse effects recorded at statistical-epidemiological levels».
It is the definitive conclusion of the study by Dr. Gabriele Segalla who dedicates an entire chapter to “ELIMINATION OF ELECTROLYTES IN THE COMPOSITION OF THE NEW MEDICINAL PRODUCT COMIRNATY TRIS”.
On 2021, October 18, EMA announced, on its official website, thatEMA’s human medicines committee (CHMP) has approved two additional manufacturing sites for the production of Comirnaty, the COVID-19 vaccine developed by BioNTech and Pfizer. One site, located in Monza,Italy, is operated by Patheon Italia S.P.A. The other in Anagni, also in Italy, is operated by Catalent Anagni S.R.L. Both sites will manufacture finished product.These sites will produce up to 85 million additional doses to supply the EU in 2021.
And, surprisingly, on the same page, it also announcedthat:
[…] The CHMP has approved a ready-to-use formulation of Comirnaty. This formulation does not require dilution prior to administration, will be available in a 10-vial (60 dose) pack size and can be stored at 2-8°C for up to 10 weeks. The current concentrated formulation requires dilution prior to Figure 20. New composition (called Tris/Sucrose Finished Product) of the Pfizer-BioNTech Comirnaty vaccine, electrolyte-free, ready to use, no longer requiring the dilution phase.
«And, on page 14 of such report, the new formulation is revealed (Figure 20), and, with it, some details that tend to confirm, both on the chemical-physical and toxicological level, the above detailed evaluation concerning the manifest instability and potential danger of the original Comirnatyflawed formulation».
Oddly enough, in the new composition of Comirnaty, called Tris/Sucrose Finished Product, containing the same active ingredient (mRNA chemically modified at nucleoside level), the same functional lipids and the same supporting excipients (at the same concentrations), all the electrolytes that were present in the previous electrolytic formulation (called, for the occasion,PBS/SucroseFinished Product, where PBS stands for Phosphate-Buffered Saline),have totally disappeared, of course without providing the reader with any explanation» the study adds
«This electrolyte purging operation may seem ordinary to non-experts, but in reality it is revealing to experts in the field of colloidal systems,andeven more explicitwhencomparing the relevant sections 3.2. of the Safety Data Sheets of Comirnaty PBS/Sucrose (the Electrolyticvaccine, Fig. 21) and Comirnaty Tris/Sucrose (the Non-electrolyticvaccine)[Figure. 22]» highlights Segalla.
It should be noted that this disrtubing research on June 13, 2022 was the subject of a parliamentary question filed by the then Italian deputy Sara Cunial to which the Draghi government, which imposed the mandatory vaccination on various professional categories in Italy, never gave an answer.
That first version of the study released in May was more integrated with paragraph 5.7 which contains the most explosive part…
THE CONSCIOUS USE OF TOXIC COMPONENTS
In the light of the technical data set outabove, it is now quite clear that the instability of the colloidal system of lipid nanomaterials(and theirconsequent greater toxicological risk) of the first version of Comirnaty is substantially due to the presence, in that formulation, of destabilizing factors, such as, in fact, the excess electrolytic inorganic compounds, which make up the PBSpH-buffer therein used by Pfizer-BioNTech».
Dr. Segalla highlights the different stabilization features of Moderna’s Spikevax competitor.
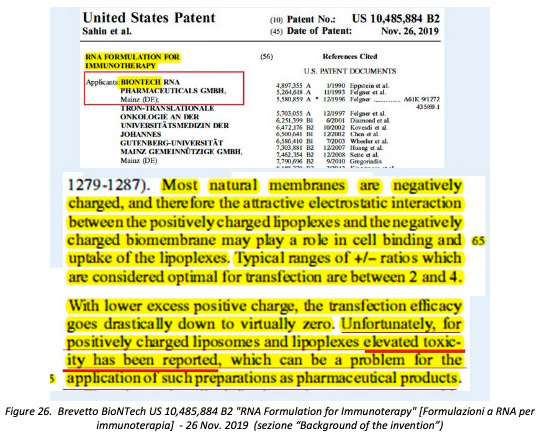
«In this same regard, however, what is reported in the patent of the sameBioNTech(co-owner, together with Pfizer, of the Comirnaty vaccine) US 10,485,884 B2RNA Formulation for Immunotherapydated November 26, 2019 is even more explicit concerning “elevated toxicity” attributed to “positively charged liposomes and lipoplexes”».
«The reference is to formulations of RNA encapsulated in cationic lipid nanoparticles —ofthe same categoryas those used in Comirnaty—and called, in this context,“lipoplexes”.In the description of the patent, it is explained, among other things, how cationic nanoparticles containing RNAare formed mainly thanks to certain mass/charge ratios between cationic (+) lipids and anionic (-) components of RNA, and how these ratios play a fundamental role also with regard to the passage of RNA-containing nanoparticles through the cell membrane and the consequent transfer of RNA inside the cell (transfection) to modify itsfunctional characteristics:
Most natural membranes are negatively charged, and therefore the attractive electrostatic interaction between the positively charged lipoplexes and the negatively charged biomembrane may play a role in cell binding and uptake of the lipoplexes. Typical ranges of+/-ratios which are considered optimal for transfection are between 2 and 4.With lower excess positive charge, the transfection efficacygoes drastically down to virtually zero. Unfortunately, for positively charged liposomes and lipoplexes elevated toxicity has been reported, which can be a problem for the application of such preparations as pharmaceutical products.[my emphasis] (Figure 26).
«The reasons why pH-buffers of the PBS-type are absolutely not suitable in preparations based on RNA-incorporating cationic nanoparticles are explained very clearly in the section Examples, Effects of Buffers/Ions on Particle Sizes and Polydispersity Index of RNA Lipoplexesof the aforementioned BioNTech patent;US 10,485,884B2, 44 (47-50), 45 (4-6), 45 (31-33)»:
The use of bufferwhich is often necessary forpharmaceutical applicationsand ions can lead to aggregation of lipoplexes which makes them unsuitable for parenteral application to patients[…]In PBS buffer, the same effect is more prominent. Lipoplexes with a positive or neutral charge ratio form larger particles (partially stabilized by the positive charges[…]Under physiological conditions(i.e. pH 7.4; 2.2 mM Ca++), a negative charge ratio appears to be imperativedue to the instability of neutral or positively charged lipoplexes. [my emphasis] (Figure 27).
«In other words, based on what is scientifically documented and reported in a patentof the same BioNTech, additionally to what already described concerning the intrinsic dangerousness of positively charged lipid nanoparticles, we learn that a colloidal system of cationic lipid nanoparticles incorporating mRNA
should NOT contain an ionic buffer such as PBS, in order to prevent aggregation, agglomeration, flocculation of lipid nanoparticles, andall the toxicological consequences described above.
should NOT contain ionic compounds(such as sodium chloride), in order to prevent aggregation, agglomeration, flocculation of lipid nanoparticles, andall the toxicological consequences described above.
should NOT be injected intramuscularly,due to its instability when placed in the physiological environment of the extracellular district (pH 7.4; 2.2 mM Ca++).
All three of these rigorous recommendations, reported in the aforementioned BioNTech patent of 2019, are shamelessly contradicted, orignored, in 2020, both by Pfizer-BioNTech and by the certifying bodies, both on the nature of the Comirnaty formulation (ionic/electrolytic) and on its intended use (intramuscular injection)».
«In the final analysis, the medicinal preparation Comirnaty/PBS Sucrose from Pfizer-BioNTech, authorized by EMA in 2020, presents serious and evident liabilitieson the chemical-physical and consequently toxicological level—liabilities, in opencontrast with the specific and pertinentrecommendationsassertedby BioNTech itself in its aforementionedpatent(US 10,485,884B2)».
THE ALLEGED CRIME DUE TO AN IMPERFECT DRUG
Based on these conclusions, integrated with the previous observations, Dr. Segalla hypothesizes…
that the addition of such an important share of electrolytic compounds to the already precarious equilibrium of a colloidal system made ofcationic nanoparticles, easilyinfluencedbyionic charges, has inevitably conditioned the stability, shelf life, functionality,and consequent toxicological potential of the finished product Comirnaty PBS/Sucrose, causing in particular: unpredictable alterations of itsPolydispersityindex and Zeta potential;
possible consequent formation of aggregates, agglomerates, flocculates, coalescences; different degrees of penetrability and mobility of nanolipid aggregates of different sizes, after inoculation, in unexpected and unpredictable biological sites, with irregularROS formation at these sites; consequent heterogeneity of adverse effects (randomization), potentially variable from batch to batch, from vial to vial, from vaccinator to vaccinator, from vaccinated to vaccinated, in a sort of ineluctable, uncontrollable,and indecipherable Russian roulette(Santiago, 2022).
Here is a possible explanation of the different lethality already found in the past in various countries with the withdrawal of some batches of the vaccine.
The “Russian roulette” was also well analyzed by a video by the same biochemist of Multichem R&D of Rozzano with a more apt title than ever in the face of a flood of even lethal adverse reactions: “Pandora’s vaccine” (link at the bottom of the article).
In the final part of it, he clarifies the concept by which Comirnaty PBS/Sucrose, being “unstable, ineffective and insecure”, can be, in Segalla’s opinion, defined as an “imperfect drug” and therefore fall within the category of crime punished by Art. 443 of the Criminal Code of the Italian Republic.
This prompted the Italian researcher to urge «an accurate and long-term study be carried out in the appropriate institutional, clinical or medico-legal seats, especially in relation to any causal or con-causal links between what is presented here and the wide pathological heterogeneity of serious or lethal adverse events that have occurred, or are occurring,after vaccinations, in order to adopt and expedite all appropriate corrective and preventive actions to protect public health,including discontinuing vaccinations with Pfizer-BioNTech Comirnaty PBS/Sucrose as soon as possible,in accordance with the precautionary principle,and in the light of Article 10 of the Nuremberg Code:
During the course of the experiment the scientist in charge must be prepared to terminate the experiment at any stage, if he has probable cause to believe, in the exercise of the good faith, superior skill and careful judgment required of him,that a continuation of the experiment is likely to result in injury, disability, or death to the experimental subject.
The judges will obviously have to say whether these assumptions of awareness of the “imperfect drug” exist which could represent the aggravating circumstance of the “possible fraud” in cases of death or damage from adverse reactions of proven correlation with the administration of Pfizer’s mRNA gene serum – Biontech.
The analysis of electrolytes and vaccine stability represents a small point in favor of Moderna which has taken a lawsuit against Pfizer precisely regarding the authorship of the revolutionary messenger RNA biotechnology.
Segalla himself has in fact noted: «the entire formulation of the new Pfizer-BioNTech preparation called Tris Sucrosebecomes, if not identical, at least very similar to that of Moderna’s Spikevax vaccine(the latter authorized by EMA on 2021, January 6, Assessment report 2021, March 11)».
On the other hand, according to the research of the American doctor David Martin, collected in his FauciDossier, the patent of the Covid vaccine of the American pharmaceutical company Moderna, financed by Bill Gates as well as by the DARPA military agency of the Pentagon, would have occurred 9 months before the discovery of SARS-Cov-2, thus corroborating the theory of the virus created in the laboratory which includes among the main probative evidence an almost identical human gene built by Big Pharma of Cambridge.
Fabio Giuseppe Carlo Carisio
Original text
Contribute a better translation